- Patients treated with LUMEVOQ® gene therapy experience better visual outcomes than idebenone-treated patients and untreated patients
- Most comprehensive analysis of visual outcomes to date among Leber Hereditary Optic Neuropathy (LHON) patients with the ND4 mutation
Paris, France, October 28, 2024, 7:30 am CET – GenSight Biologics (Euronext: SIGHT, ISIN: FR0013183985, PEA-PME eligible), a biopharma company focused on developing and commercializing innovative gene therapies for retinal neurodegenerative diseases and central nervous system disorders, today announced the publication of meta-analyses comparing visual outcomes among patients with Leber Hereditary Optic Neuropathy (LHON) caused by a mutation in the MT-ND4 mitochondrial gene (ND4-LHON), the most common mutation leading to the poorest visual prognosis[1].
The paper, published in the peer-reviewed journal Survey of Ophthalmology, is the first to compare the efficacy of LHON treatments, approved or in development, on visual outcomes in the ND4-LHON patient population and to compare these outcomes with those in untreated (natural history) patients. The meta-analyses establish a “gradient of efficacy” in two measures of visual outcomes assessed in the paper, with LUMEVOQ® gene therapy having better outcomes compared to idebenone treatment and both treatments having better outcomes compared to the natural history of the disease.
As measured by the rate of Clinically Relevant Recovery (CRR)2, which is the responder rate common across the studies analyzed in the paper, the rate of visual recovery after LUMEVOQ® gene therapy is triple that in the natural evolution of ND4-LHON and substantially greater than that among idebenone-treated patients.
“This study is very important for comparing these therapies’ abilities to achieve visual function outcomes in ND4-LHON patients” commented Dr. Nancy J. Newman, LeoDelle Jolley Professor of Ophthalmology and Neurology at the Emory University School of Medicine in Atlanta, United-States, and lead author. “For the first time, we clearly demonstrate a gradient of efficacy in visual outcomes, more marked for Clinically Relevant Recovery (CRR) than for final Best-Corrected Visual Acuity (BCVA), with lenadogene nolparvovec gene therapy superior to idebenone treatment, and both superior to the natural history of the disease.”
The article updates an earlier set of findings, presented at the 2024 North American Neuro-Ophthalmology Society annual meeting, by including data from the recently published LEROS trial for idebenone and by including an assessment of final Best-Corrected Visual Acuity (BCVA) outcomes among the three groups of patients.
Figure 1. “A Gradient of Efficacy”: Visual Recovery (CRR[2] from Nadir[3]) Among ND4-LHON Patients[4],[5]
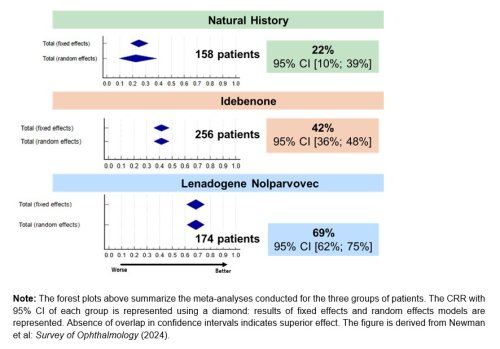
The meta-analyses are the largest comprehensive review of the available data on the natural history of patients with vision loss from the MT-ND4 LHON mutation and those treated with idebenone, as well as all the available results on the efficacy of lenadogene nolparvovec gene therapy. The ND4-LHON population included in each group was representative of the typical demographics of the disease as reported in a century and a half of literature. In the natural evolution of the disease, most ND4-LHON patients experience a severe and chronic reduction in visual acuity in both eyes, which seriously affects their quality of life.
The article is available online via this link.
[1] Newman et al: J Neuro-Ophthalmol 2020 Dec;40(4):547-557.
[2] “Clinically Relevant Recovery”, or CRR, denotes an improvement in Best-Corrected Visual Acuity (BCVA) that satisfies one of two conditions: (1) A 10-letter (≥0.2 LogMAR) improvement for an on-chart starting visual acuity. (2) Improvement from “off-chart” to “on-chart” (≤1.6 LogMAR).
[3] Nadir = lowest observation of visual acuity between baseline and time point of interest.
[4] The three meta-analyses were pre-defined in a statistical analysis plan
[5] Sources of data: For idebenone and natural history data, systematic review of the literature and available clinical/regulatory reports on MT-ND4 LHON patients; for Lumevoq, all phase 3 studies (RESCUE/RESTORE, REVERSE/RESTORE and REFLECT).
Contacts
-
GenSight BiologicsChief Financial OfficerJan Eryk Umiastowski
-
LifeSci AdvisorsInvestor RelationsGuillaume van Renterghem+41 (0)76 735 01 31